WHAT YOU NEED TO KNOW ABOUT THE FLU AND COVID-19 VACCINES
With 2020 finally over, a new worry carries over. Should you get the flu vaccine? Is the COVID-19 safe vaccine?
With COVID-19 cases surging, the vaccine is a guaranteed way to avoid the virus.
As we embrace these chillier months and flu season, questions arise on whether the flu shot or COVID-19 shot is right for you.
We’re here to fill you in on everything you need to know about the flu, Pfizer-BioNtech, and the Moderna vaccines.
The Flu Shot
Centers for Diseases Control and Prevention (CDC) reports, “There are many different flu viruses and they are constantly changing. The composition of U.S. flu vaccines is reviewed annually and updated as needed to match circulating flu viruses.” This means it’s completely safe, and you should be getting your flu shot every year to prevent contracting the virus. “For the 2020-2021 season, manufactures have projected they will provide as many as 194-198 million doses.” There are different flu shots available based on age group: inactivated influenza vaccines (IIV), recombinant influenza vaccine (RIV), and high-dose inactivated vaccines.

-Inactivated (IIV) is approved for people as young as 6 months of age.
-Recombinant (RIV) is approved for people 18 years and older.
-Fluzone High-Doze Quadrivalent is approved for people 65 and older.
Like with any medication, vaccines also have health risks and concerns. While rare, there are cases where individuals should NOT receive the flu shot. The CDC warns:
-Children younger than 6 months are too young for the shot.
-People with severe, life-threatening allergies to the flu vaccine or any ingredient in the vaccine. This includes gelatin, antibiotics, and more.
-People with a history of Guillain-Barre Syndrome (GBS).
For additional information regarding egg allergies and the flu vaccine, please visit Special Considerations Regarding Egg Allergy.
An alternative to the injectable flu vaccine is the nasal flu vaccine. The nasal spray vaccine is approved for use in healthy non-pregnant individuals, 2 to 49 years of age.

Center for Infectious Disease Dynamics (CIDD) states, “Getting a flu shot this year is particularly important because, like SARS-CoV-2 [pubiclly known as COVID-19], influenza is a respiratory virus… it’s also possible that with one infection, it could weaken the immune system and increase susceptibility to another.”
The Flu and COVID-19
Are you unsure of the difference between the flu and COVID-19? As stated on the CDC webpage, “both are contagious respiratory illnesses” that are caused by different viruses. Influenza, [commonly known as flu], is caused by the influenza virus, while COVID-19 is caused by a coronavirus [widely known as SARS-CoV-2].
People who fear having contracted the COVID-19 virus experience similar symptoms as the flu, which makes it difficult to diagnose without proper testing. The CDC confirms, “It is possible to have the flu, as well as other respiratory illnesses, and COVID-19 at the same time.” These are the notable differences between the flu and COVID-19:
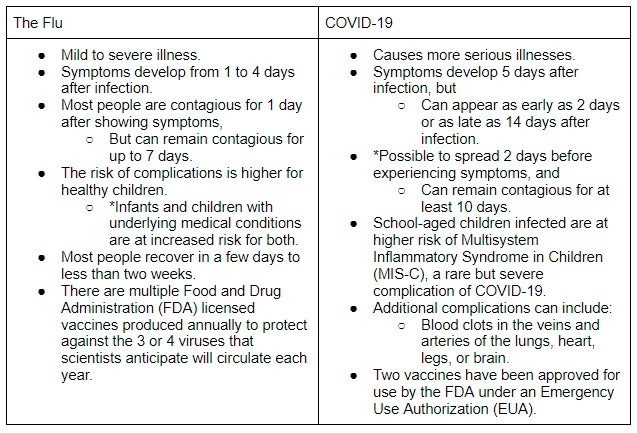
With this list of differences, you might feel inclined to make an appointment for both vaccines. The CDC suggests, “you should not get [them] at the same time. COVID-19 vaccines should be given alone with at least 14 days either before or after you get any other vaccines.”
Pfizer-BioNtech and the Moderna Vaccines
When news about the COVID-19 outbreak happened, the public was concerned for their health and wellbeing. Fast-forward a year, and now two vaccines are available. It’s understandable to raise an eyebrow at the speed of this vaccine. You might wonder how safe it is.
Lisa Maragakis, M.D., M.P.H., senior director of infection prevention, and Gabor Kelen, M.D., director of the John Hopkins Office of Critical Event Preparedness and Response, clarify some of those concerns.

-All vaccines go through clinical trials to test safety and effectiveness.
-When it comes to authorization for emergency use, vaccines that meet the FDA safety and effectiveness standards can be made available in the United States by approval.
-Once a vaccine is authorized for use, monitoring continues, with systems in place to track problems or side effects that were not detected during the clinical trials.
-Pfizer-BioNtech has been years in development for other infectious viruses. Thus, the manufacturing process was ready very early in the pandemic.
-Both manufacturers report that their vaccines show approximately 95% efficacy at preventing both mild and severe symptoms of COVID-19.
While it might take longer for the general public to receive the COVID-19 vaccines, you can help flatten the curve by wearing a mask, socially distancing, disinfecting surfaces, and avoiding gatherings.
We hope you found this information helpful. Whether you decide to talk to your health care provider regarding the COVID-19 or Flu vaccine, remember to wait at least two weeks before receiving additional vaccinations. As always, stay safe and charming!